Radiumo
88
Ra
Grupo
2
Perio
7
Bloko
s
Protonoj
Elektronoj
Neŭtronoj
88
88
138
Ĝeneralaj Propraĵoj
Atoma Nombro
88
Atommaso
[226]
Amasa Nombro
226
Kategorio
Teralkalaj metaloj
Koloro
Arĝento
Radioaktiva
Jes
From the Latin word radius meaning ray
Kristala Strukturo
Korpo Centrita Kubo
Historio
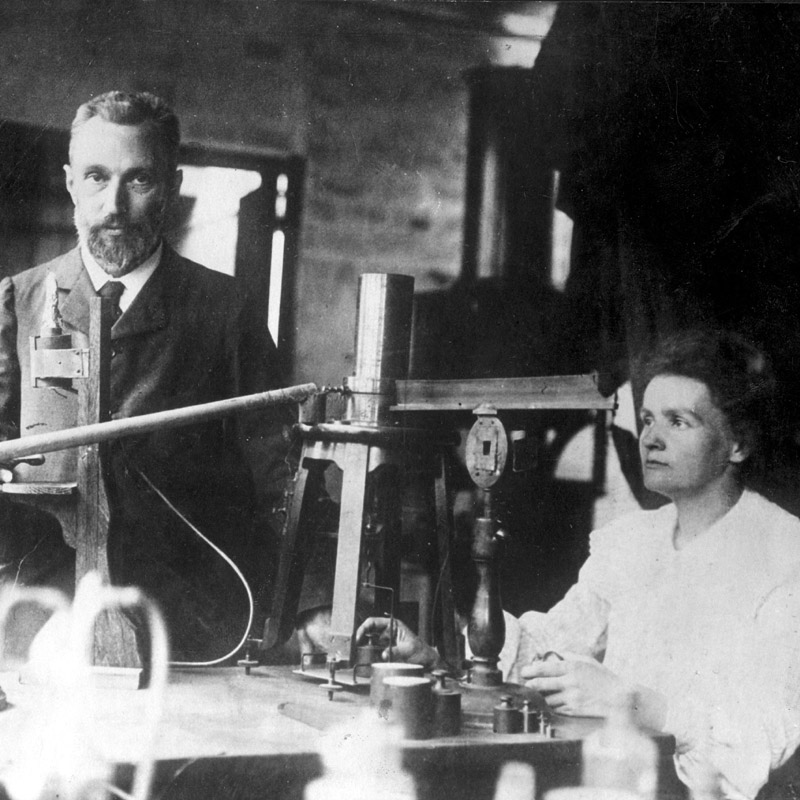
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Elektronoj per ŝelo
2, 8, 18, 32, 18, 8, 2
Elektrona Agordo
[Rn] 7s2
Radium imparts a carmine red color to a flame
Fizikaj Propraĵoj
Fazo
Solido
Denso
5,5 g/cm3
Fandpunkto
973,15 K | 700 °C | 1292 °F
Bolpunkto
2010,15 K | 1737 °C | 3158,6 °F
Varmo de Fuzio
8 kJ/mol
Varmo de Vaporiĝo
125 kJ/mol
Specifa Varmo Kapacito
- J/g·K
Krusta Abundo
9,9×10-12%
Universa Abundo
Neniu
CAS Nombro
7440-14-4
PubChem Kunmetita Identiga Nombro
6328144
Atomaj Propraĵoj
Atoma Radiuso
-
Kovalenta Radiuso
221 pm
Elektronegativeco
0,9 (Pauling-skalo)
Potencialo de jonigo
5,2784 eV
Atoma Volumo
45,20 cm3/mol
Termika Kondukto
0,186 W/cm·K
Oksidaj Ŝtatoj
2
Aplikoj
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium is highly radioactive and carcinogenic
Izotopoj
Stabilaj Izotopoj
-Malstabilaj Isotopoj
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra