Polonio
84
Po
Grupo
16
Perio
6
Bloko
p
Protonoj
Elektronoj
Neŭtronoj
84
84
126
Ĝeneralaj Propraĵoj
Atoma Nombro
84
Atommaso
[210]
Amasa Nombro
210
Kategorio
Metaloidoj
Koloro
Arĝento
Radioaktiva
Jes
Named after Poland, native country of Madam Curie
Kristala Strukturo
Simpla Kubo
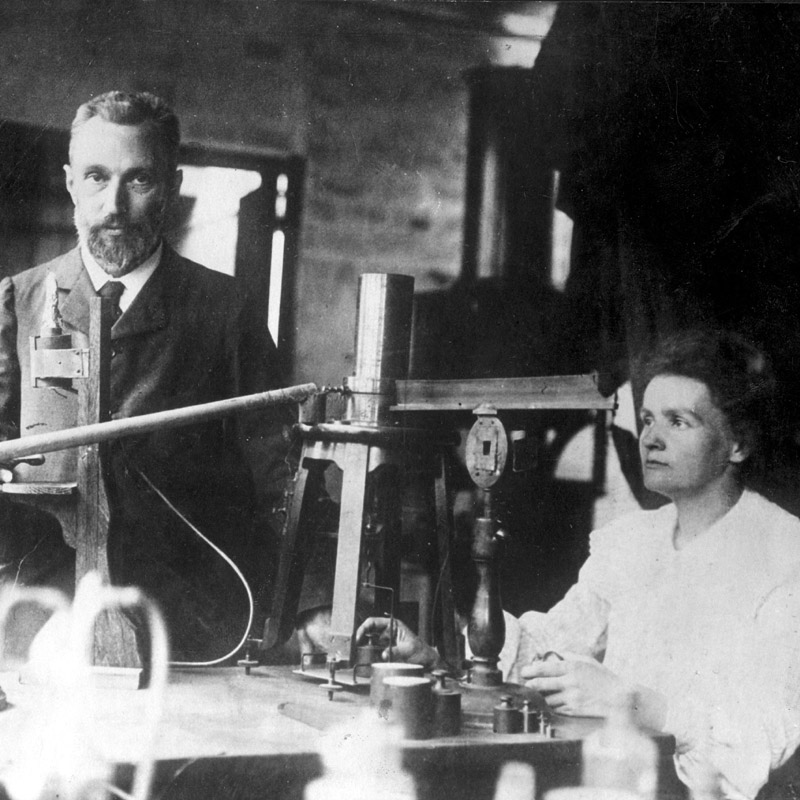
Historio
Polonium was discovered by Marie and Pierre Curie in 1898 in Paris.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
Elektronoj per ŝelo
2, 8, 18, 32, 18, 6
Elektrona Agordo
[Xe] 4f14 5d10 6s2 6p4
Polonium is obtained by irradiating bismuth with high-energy neutrons or protons
Fizikaj Propraĵoj
Fazo
Solido
Denso
9,196 g/cm3
Fandpunkto
527,15 K | 254 °C | 489,2 °F
Bolpunkto
1235,15 K | 962 °C | 1763,6 °F
Varmo de Fuzio
13 kJ/mol
Varmo de Vaporiĝo
100 kJ/mol
Specifa Varmo Kapacito
- J/g·K
Krusta Abundo
Neniu
Universa Abundo
Neniu
CAS Nombro
7440-08-6
PubChem Kunmetita Identiga Nombro
Neniu
Atomaj Propraĵoj
Atoma Radiuso
168 pm
Kovalenta Radiuso
140 pm
Elektronegativeco
2,00 (Pauling-skalo)
Potencialo de jonigo
8,417 eV
Atoma Volumo
22,23 cm3/mol
Termika Kondukto
0,2 W/cm·K
Oksidaj Ŝtatoj
-2, 2, 4, 6
Aplikoj
Polonium is used to eliminate static electricity produced during processes such as rolling paper, wire and sheet metal.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium is highly dangerous and radioactive
Izotopoj
Stabilaj Izotopoj
-Malstabilaj Isotopoj
188Po, 189Po, 190Po, 191Po, 192Po, 193Po, 194Po, 195Po, 196Po, 197Po, 198Po, 199Po, 200Po, 201Po, 202Po, 203Po, 204Po, 205Po, 206Po, 207Po, 208Po, 209Po, 210Po, 211Po, 212Po, 213Po, 214Po, 215Po, 216Po, 217Po, 218Po, 219Po, 220Po